TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a 25 oC HClO 4 ClO 4 H 2 SO 4 HSO 4 HCl Cl HNO 3 NO 3 H 3 O H 2 O H 2 CrO 4 HCrO 4 18 x 101 H 2 C 2 O 4 oxalic acid HC 2 O 4 590 x 102 H 2 SO 3 SO 2 aq H 2 O HSO 3 171 x 102 HSO 4 SO 4 2 120 x 102 H 3 PO 4 H 2 PO 4 752 x 103 FeH 2 O 6 3 FeH 2 O 5 OH 2 184 x 103 H 2 C 8 H. That is because sulfuric acid is a strong acid and completely disassociates in water.
Solved Identify The Conjugate Base For Each Acid Er Chegg Com
Chemistry questions and answers.
. A more general definition is that a conjugate base is the base member X- of a pair of compounds that transform into each other by gaining or losing a proton. We see that HCO₃ becomes H₂CO₃. A HSO4- -- SO4-2.
Conjugate acid of HSO4. So H₂CO₃ is the conjugate acid of HCO₃. Solution for Identify the conjugate acid for each base.
Typo in question Answer is wrong. The H₂O becomes OH. First week only 499.
When this acid donates an. Although it has a negative charge it will never accept a H to form H 2SO4 sulfuric acid. A Identify the conjugate base for each acid.
Weve got the study and writing resources you need for your assignments. The conjugate base is able to gain or absorb a proton in a chemical reaction. Thus for the ionization of HCl HCl is the conjugate acid and Cl is the conjugate base.
So OH is the conjugate base of H₂O. In an acid-base reaction the chemical. K2HASO3aq CBr3COOKaq K3ASO3aq.
C Conjugate base of HCN is. Write the chemical formula for the conjugate base of each of the following weak acids. Part A HNO2 Express your answer as a chemical formula.
Identify the conjugate acid for each base. Conjugate acid of PO. Conjugate acid of NH3.
Create a New Plyalist. Bromous acid 111 Match the formulas of acids and bases with their names. Write the formula for the conjugate acid for each of the following bases.
Acid is defined as a substance which looses donates protons and thus forming conjugate base. Conjugate acid of SO24. Study with Quizlet and memorize flashcards terms like 111 Select the correct name for each of the following acids.
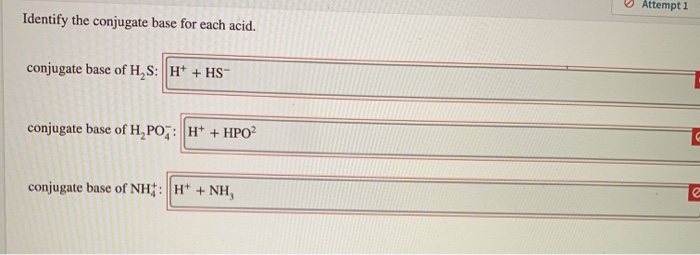
Acids have one more H ion than there conjugate base pairs so to write a conjugate base pair simply remove a H and subtract a charge. HCl aq H 2 O H 3 O aq Cl aq In the discussion of Brønsted acid-base behavior the hydrogen atom that is transferred is generally referred to as a proton because it is transferred as a hydrogen atom without its electron. Conjugate base of H2PO4.
This now can serve as a base. Every time a Brnsted acid acts as an H -ion donor it forms a conjugate baseImagine a generic acid HA. The conjugate base of the methylammonium ion is CH3NH2 methylamine.
Conjugate base of NH4. Add to Existing Playlist. It has one less H atom and one more charge.
Base acid Conj A Conj B. It has one more H atom and one more charge -1 1 0. By doing so it makes the molecule more negative.
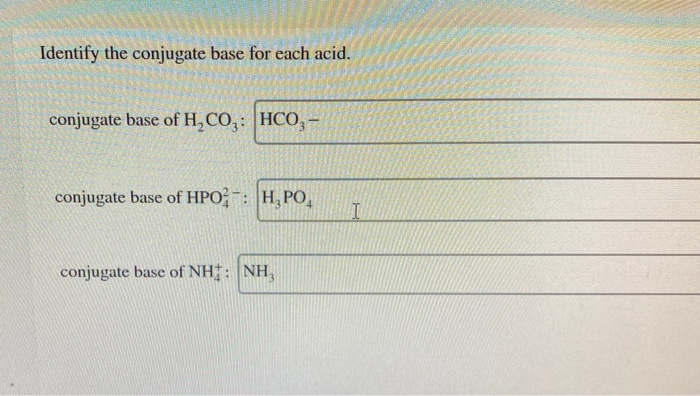
Conjugate base of H2CO3. Conjugate base of HPO24. Part A HNO2 Express your answer as a chemical formula.
The conjugate acid donates the proton or hydrogen in the reaction. In aqueous solution the methylammonium ion acts as a weak acid. The water molecule acts as bases.
Identify the conjugate base for each acid. It could accept a hydrogen ion. Write the formula for the conjugate base of each acid.
A Conjugate base of HCl is. Therefore the sulfate ion SO2 4 is the conjugate base of H SO 4. B Conjugate base of is.
Start your trial now. Chemistry questions and answers. B At 25 C what is the hydroxide ion concentration OH in an aqueous solution with a.
So one conjugate acid base pair is H s 04 minus and s 04 to minus s 04 to minus is the conjugate base to H S 04 minus and H S 04 minus is the conjugate acid Toso four minus. Acids and bases exist as conjugate acid-base pairsThe term conjugate comes from the Latin stems meaning joined together and refers to things that are joined particularly in pairs such as Brnsted acids and bases. D Conjugate base of HF is.
Enter Friends Emails Share Cancel. Notice that you can reform the methylammonium ion by adding a proton to its conjugate base. Write the formula for the conjugate base of each of the following acids.
Conjugate acid of S2. Solution for For each of the following equations identify the acid base conjugate acid and conjugate base. To determine the conjugate base remove a hydrogen atom and remove a unit of.
CH3NH 3 CH3NH2 H. Write the formula for the conjugate base of each acid. 3 _3- 3.
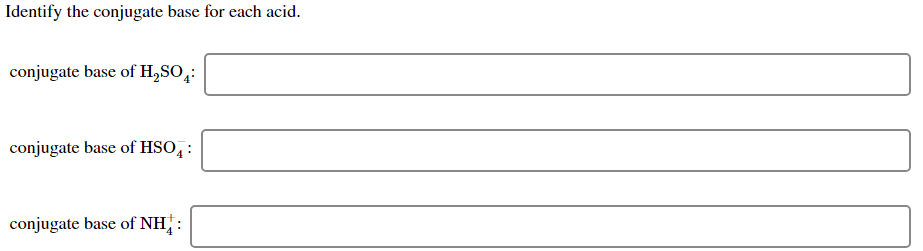
Conjugate acid of HSO4. All you need to do is remove a proton H. Conjugate acid of NH.
H2OlH3Oaq H3O is an acid and H3O is a conjugate base. So in the reverse reaction this is the base. C HPO42- -- PO4-3.
Conjugate base of H2S. To determine the conjugate base remove a hydrogen atom and remove a unit of positive charge from the chemical formula. Base is defined as a substance which accepts protons and thus forming conjugate acid.
Conjugate acid of HSO. Identify the conjugate acid for each base. SO4-2 OH-1 HS-1 ClO2-1.
Keep in mind that as you remove a proton it gives electrons to the conjugate base. Write the formula for the conjugate base of each of the following acids. According to the Bronsted-Lowry conjugate acid-base theory.
Write the conjugate base for each of the following acids.

Solved Identify The Conjugate Base For Each Acid Conjugate Chegg Com

Solved Identify The Conjugate Base For Each Acid Conjugate Chegg Com

Solved Identify The Conjugate Base For Each Acid Conjugate Chegg Com
Solved Identify The Conjugate Base For Each Acid Conjugate Chegg Com
0 Comments